Research Facility
VESO Aqualab
is a contract research facility specializing in trials with infectious diseases in fish. We offer standardized challenge models for Atlantic salmon, rainbow trout, seabass and lumpfish, and may contribute to develop models for other species. Pathogens include viruses, bacteria and parasites for well-known as well as emerging diseases. VESO Aqualab offers long-term vaccine studies (duration of protection) and field trials conducted under commercial farming conditions.
VESO Aqualab represents an independent contract research facility, always ensuring full confidentiality and customer dedication. Our clients are the pharmaceutical-, feed- and breeding industries, research institutions and government bodies. We focus on trial quality systems (GMP, GLP and GCP) and strive to maintain the highest ethical profile in all our activities.
Our aim has been to make the VESO trademark synonymous with uncompromising quality and reliability in fish health research.
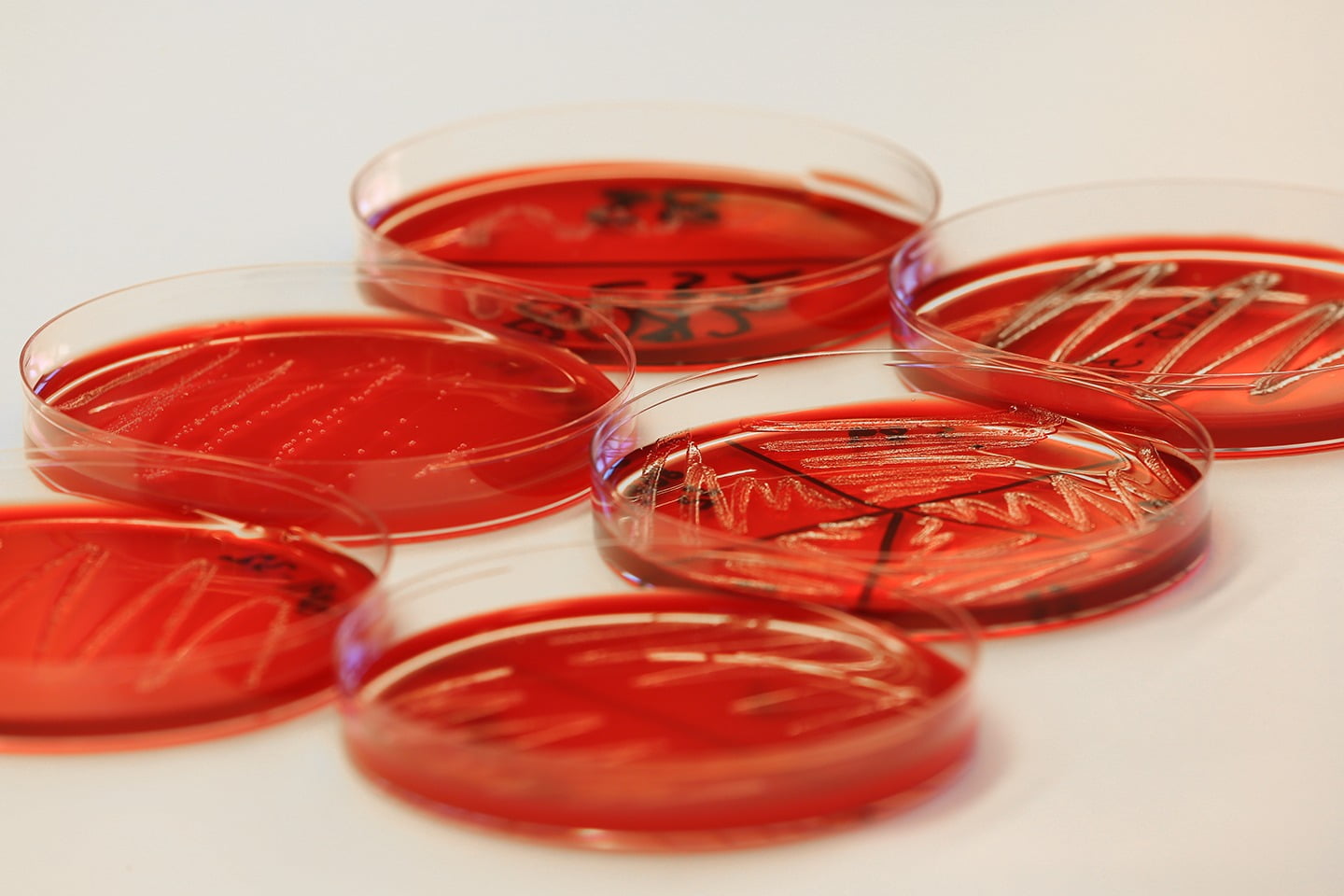
Experimental challenge trials
Challenge experiments are designed to evaluate the performance of vaccines and pharmaceuticals, effects of health feeds and genetic resistance profiles against infectious agents. VESO Aqualab’s R&D focus is to develop and maintain reliable and clinically relevant challenge models for pathogens affecting global aquaculture, especially salmonids. VESO Aqualab’s highly qualified and experienced staff works in close collaboration with research institutions and the aquaculture industry. We subcontract expertise and services on behalf of our customers when necessary to ensure full width documentation of test products, and to ensure full compliance with formal test requirements. We are happy to develop new models and standards in collaboration with our clients.
Quality assurance
VESO Aqualab is certified according to GLP (Good Laboratory Practice) and holds a manufacturing license for medicinal products according to GMP (Good Manufacturing Practice) requirements. Trials in the laboratory and field can be performed according to GCP (Good Clinical Practice). We also offer monitoring services for trials run according to GCP at independent trial locations.
The facility
VESO Aqualab is a state-of-the-art non-recirculation trial unit lab with advanced registration and surveillance systems. The research facility consists of a wet lab with separate isolates and holding tanks from 5 to 13 000 liters. The tank design can be customized to accom-modate research needs, including individual light and feeding regimes, salinity and temperature. The research facility has two fresh water sources and sea water is drawn from depths down to 60 meters outside the facility. The salinity of the fjord water is ~33 ‰. Incoming water is filtered and UV-treated prior to admittance to the test area. Effluent water is filtered and disinfected with sodium hypochlorite and ozone before release to the recipient.
Clinical field trials
VESO Aqualab holds two salmon farming licenses for research purposes. The licenses enable trial conduct under commercial farming conditions throughout the salmon life cycle from hatching to harvest. The key application for this composite trial system is to enable evaluation of efficacy and side effects of vaccines and pharmaceuticals in a real-life environment, and to facilitate clinical validation of our laboratory challenge models. Trials are routinely conducted according to GCP (Good Clinical Practice).


Production of fish
Atlantic salmon for trials are produced at VESO Aqualab’s hatchery. The hatchery is certified according to GLOBALG.A.P. standards. Production of our own test fish ensures quality in every step from egg to seaworthy smolts. The hatchery produces Atlantic salmon all year round and our fish are certified pathogen free before being used in trials.
Staff
The scientific staff at VESO Aqualab comprises fish health biologists, veterinarians and university graduates (Msc or PhD). The technical staff is educated in aquaculture. All employees are trained to conduct trials according to the principles of GLP (Good Laboratory Practice).
Research Facility
VESO is positioned to run experimental challenge trials in a separate unit of the Cargill Innovation Centre in Colaco, Chile. Test fish are produced on site, and trials are performed according to the same high quality standards as in Norway. We have a dedicated staff trained according to VESO procedures who design and perform trials according to the clients’ needs.
The VESO Organisation
VESO (Centre of Veterinary Contract Research and Commercial Services Ltd) was established in 1988. VESO consists of VESO Aqua (distribution of vaccines and pharmaceuticals to the aquaculture industry), VESO Hygiene (high quality washing and disinfection products for aquaculture and agriculture), VESO Aqualab (contract research facilities located in Norway and Chile), and Brynsløkken (antifouling products).


